In numerous scientific fields, high-throughput experimentation methods combined with artificial intelligence (AI) show great promise to accelerate innovation and scientific discovery. Case in point: In just five months, researchers at the U.S. Department of Energy’s (DOE) Argonne National Laboratory used robotics, automation and AI to conduct more than 6,000 experiments on chemicals in a type of rechargeable energy storage called organic redox flow batteries (RFBs). Such a monumental effort would have taken five to eight years with traditional experimentation.
Organic RFBs use carbon-based — that is, organic — molecules instead of traditional metal ions. Through their work, the researchers made a crucial finding about these batteries: A fundamental barrier at the molecular level limits their stability. The insight is expected to inspire exciting new directions in battery chemical research.
“This study would not have been possible without our high-throughput methods,” said Lily Robertson, Argonne assistant chemist and lead author of the study. “It provides the global battery community with a much deeper understanding of the factors governing stability in organic redox flow batteries. This understanding opens a door for battery developers to reimagine how this technology can support the energy system.”
The study, published in the Journal of the American Chemical Society, was conducted at one of Argonne’s Robotic Autonomous Platforms for Innovative Discovery (RAPID) laboratories used for studying chemistry and materials problems including those in energy storage, critical materials, microelectronics and quantum systems. It is one of several prototype laboratories established as part of Argonne’s wide-ranging efforts to advance autonomous discovery.
“Pairing artificial intelligence with robotics is transforming the way we do science,” said Kawtar Hafidi, associate laboratory director for Physical Sciences and Engineering at Argonne. “By enabling autonomous discovery, we can move from years of trial and error to rapid, data-driven breakthroughs that keep the United States at the forefront of scientific innovation and global competitiveness.”
Shedding light on an invisible stability barrier
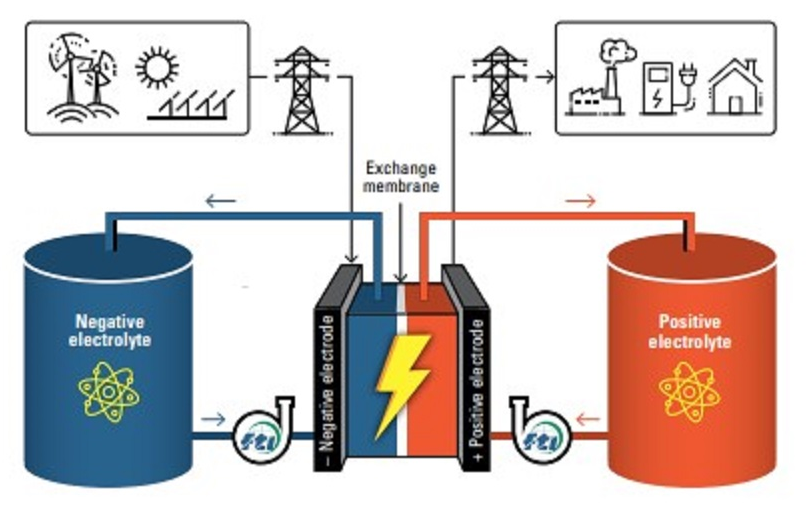
RFBs are typically composed of three constituents: a liquid solvent, charged molecules and a supporting salt. The charged molecules and salt are dissolved in the solvent, which is kept in tanks. During battery operation, the liquid is pumped through an electrochemical cell stack where the charged molecules are oxidized and reduced. These reactions can either generate or store electricity. The salt provides conductivity and helps maintain an optimal distribution of charges.
Organic RFBs have long been proposed as a promising solution to provide large-scale energy storage to reinforce the electricity grid. Unlike the lithium-ion chemistries used in other battery technologies, the compounds used in organic RFBs are abundant and inexpensive. This makes them potentially more cost-effective and scalable for long-duration energy storage. Additionally, organic RFBs can potentially operate at higher voltages than RFBs made with water-based materials. This enables them to pack more energy into the same amount of space.
For many years, researchers around the world have tried hard to improve the performance of organic RFBs. While progress has been made, a stubborn challenge remains: insufficient stability for long-term operations.
The charged molecules tend to be reactive, particularly at higher voltages. When a charged molecule reacts, its charge typically cannot be recovered. Reactions cause the battery materials to degrade and lose their storage capacity. This degradation has made organic RFBs unsuitable for long-duration, grid-scale energy storage, which requires many years of stable operation.
“The research community has run up against an invisible stability barrier,” said Ilya Shkrob, Argonne chemist and co-author of the study. “This suggests that something fundamental has been missing from our understanding of how charged organic molecules behave in these batteries.”
The Argonne study’s goal was to test the existence of this barrier. To do this, the team sought to answer a very broad question: Can selecting the right solvent significantly increase the stability of the charged molecules?
With the traditional, human-driven experimental approach, tackling such an ambitious research question would require years of coordinated global effort to investigate the vast space of organic solvents. Laboratory automation offered an intriguing opportunity to address the challenge in a much shorter timeframe and with substantially fewer resources.

Charting the chemical landscape
As an initial task in the study, the researchers sought to pinpoint the reactions that limit the longevity of the charged molecules. They used nuclear magnetic resonance spectroscopy to characterize the evolution of charged molecules called methylphenothiazine (MPT) when mixed with solvents.
“We found that the charged molecules were fragmenting the solvent molecules and getting neutralized,” said Robertson.
Next, the Argonne team determined how quickly MPT reacted with low concentrations of numerous solvents selected from a chemical database, industrial solvent lists and commercial catalogs.
Because these materials are sensitive to oxygen and moisture, the researchers set up a liquid-handling robot in an air-free glovebox chamber. The robot prepared the MPT-solvent mixtures and assembled them on microplates, each containing 384 wells. Two plate reader spectrophotometers tracked the color change of the samples as the charged molecules decayed. This revealed the reaction rates, which were compared with the reaction rate associated with a baseline solvent.
“Our finding was sobering,” said Shkrob. “Most solvents followed similar degradation pathways.”
Slower-reacting solvents were then tested at high concentrations with and without a liquid salt. Ultimately only three solvents significantly outperformed the baseline.
Machine learning algorithms guided the test iterations based on analysis of the experimental data, eliminating unnecessary experiments. This allowed the team to characterize 540 solvents by sampling just a third of them.
Promising new research avenues
Up until this study, researchers have focused on finding extremely long-lasting organic charged molecules for RFBs. By demonstrating that this may be an unrealistic goal, the study is likely to spark fresh thinking about new research directions and novel battery deployment strategies.
For one, the most stable solvents discovered in the study may work better in battery technologies like sodium-ion and lithium metal, which require stability at high-voltage operation. Other researchers can use Argonne’s high-throughput experimentation approach to explore this possibility.
Alternatively, the study’s insights may encourage the development of innovative use cases to make organic RFBs commercially viable. For instance, organic materials could be used in grid-scale batteries for a limited time and then repurposed for other applications, such as agricultural herbicides and materials for the chemical industry.
Argonne is a leader in the use of autonomous discovery methods to drive innovation in fundamental science. Its advancements in self-driving laboratories will continue to drive breakthroughs in energy storage, catalysis, materials science, quantum systems and biological processes.
Besides Robertson and Shkrob, the other authors of the study are Ryan Lewis, Rafael Vescovi and Benjamin T. Diroll. Additional contributors include Noah Paulson, Tobias Ginsburg, Casey Stone, Magali Ferrandon, Zhengcheng Zhang, and Max Delferro from Argonne, and Jeffrey Moore of the University of Illinois Urbana-Champaign.
This research was supported by Laboratory Directed Research and Development funding from Argonne. Work performed at the Center for Nanoscale Materials, a DOE Office of Science user facility, was supported by the DOE Office of Basic Energy Sciences.