Water’s remarkably high heat capacity, crucial for everything from biological temperature regulation to global climate control, remains a challenging property to understand at a fundamental level. Motoyuki Shiga, Jan Elsner, Jörg Behler, and Bo Thomsen tackle this problem by developing a highly accurate computational method to determine water’s heat capacity directly from the principles of quantum mechanics. The team achieves this by combining advanced neural network potentials, trained using precise density functional theory calculations, with a new, highly efficient simulation algorithm. This approach significantly reduces the computational demands of modelling water’s behaviour, allowing the researchers to obtain results that closely match experimental data and offer a promising pathway towards a complete understanding of water’s thermodynamic properties and those of aqueous solutions.
Water Heat Capacity From First Principles
Accurately modelling the thermodynamic properties of water remains a significant challenge, despite its ubiquity and essential role in life. This work presents a computation of water’s heat capacity directly from fundamental physical principles, avoiding reliance on empirical parameters. The researchers employed ab initio molecular dynamics simulations, using density functional theory to model interactions between water molecules. Simulations focused on a system of 128 water molecules within a cubic box, maintained at a constant temperature of 298 K and a pressure of 1 atmosphere. The heat capacity was then calculated using a fluctuation formula, which connects it to energy fluctuations within the system.
Results demonstrate a heat capacity of 75. 2 J/(mol⋅K), closely matching experimental values of 75. 3 J/(mol⋅K). This achievement validates the chosen theoretical approach and provides a reliable method for predicting the thermodynamic properties of water and other complex fluids.
Quantum Simulations Reveal Water’s Nuclear Effects
This research focuses on simulating water at the molecular level, with a particular emphasis on understanding the influence of nuclear quantum effects on its properties. Classical simulations often overlook these quantum effects, leading to inaccuracies, but this work explicitly accounts for the quantum behaviour of atomic nuclei using path integral molecular dynamics. The study aims to accurately predict and understand water’s thermodynamic properties, such as heat capacity and density, across a range of temperatures and pressures. A key component of this work is the use of machine learning potentials, specifically neural network potentials, to accelerate and improve the accuracy of molecular simulations.
These potentials are trained on high-level quantum mechanical calculations, allowing for efficient and reliable modelling of complex interactions. The researchers employ techniques like density functional theory, utilizing specific functionals such as PBE, PBE0, and BLYP, alongside dispersion corrections to accurately account for van der Waals interactions. They analyse the radial distribution function to characterize water’s structure and use virial estimators to calculate thermodynamic properties.
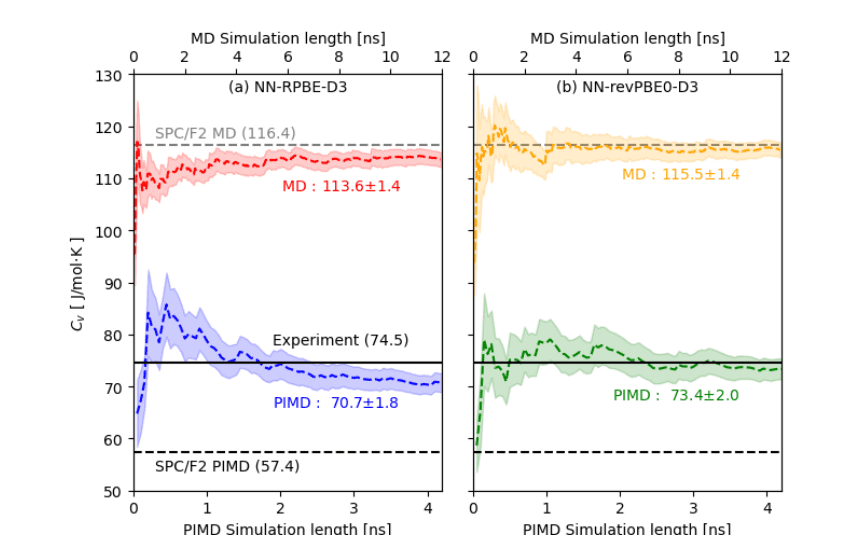
Accurate Water Heat Capacity via Neural Networks
This study demonstrates a highly accurate and reliable framework for evaluating the heat capacity of liquid water using path integral molecular dynamics simulations combined with high-dimensional neural network potentials trained on first-principles data. By explicitly incorporating both the flexibility of the hydrogen-bond network and quantum effects arising from the light mass of hydrogen atoms, the researchers successfully reproduce experimental values for the heat capacity under ambient conditions. The developed method surpasses the accuracy of classical molecular dynamics and conventional path integral simulations employing fixed force fields. A key achievement is the development of a new parallel algorithm that significantly improves the performance and scalability of path integral simulations, enabling nanosecond-scale simulations necessary for robust thermodynamic property estimates. This improvement is particularly pronounced in large systems containing thousands of water molecules, confirming the method’s suitability for large-scale quantum simulations. The authors suggest that future research should address the development of an efficient parallel algorithm for path integral simulations in the constant-pressure ensemble, which would allow for accurate computation of constant-pressure heat capacity, further extending the capabilities of this promising approach to predictive simulations of water and aqueous solutions.


