Zinc battery performance and durability have been significantly enhanced by a novel electrode protective layer developed by a joint research team from Korea University and Seoul National University.
Led by Professor Yu Seung-ho at KU’s Department of Chemical and Biological Engineering, in collaboration with Professor Sung Yung-eun’s group, the study has been published online in Advanced Functional Materials.
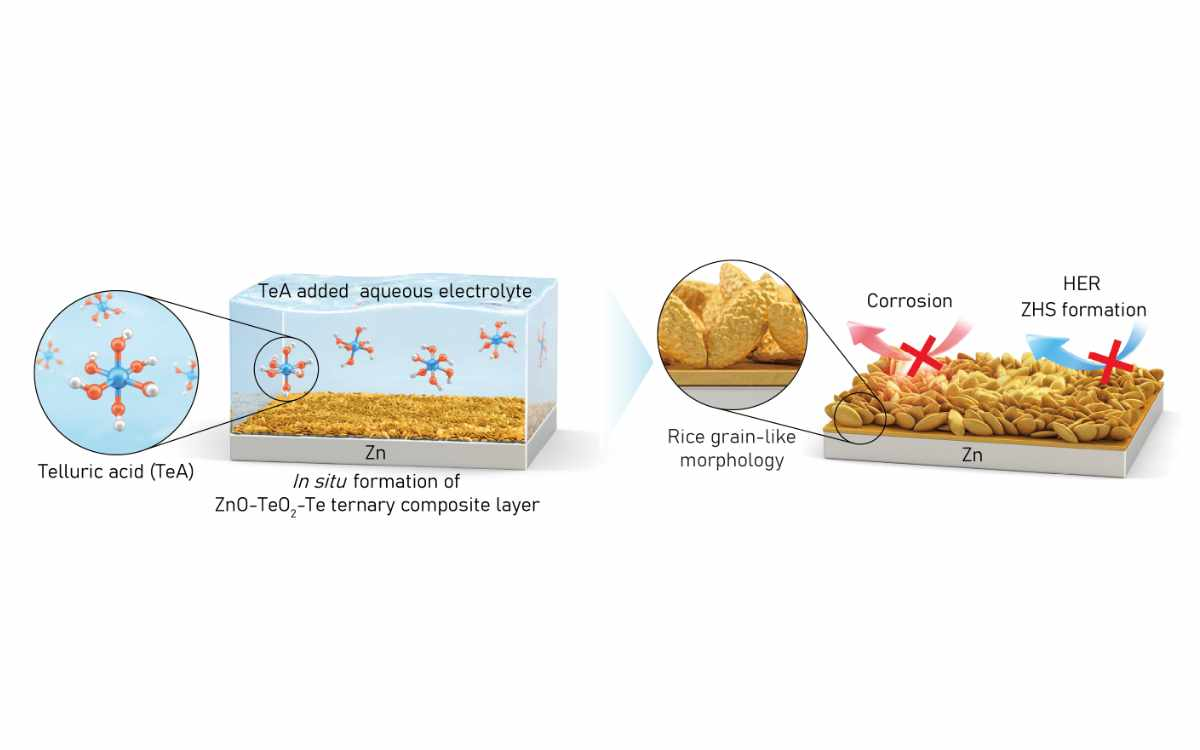
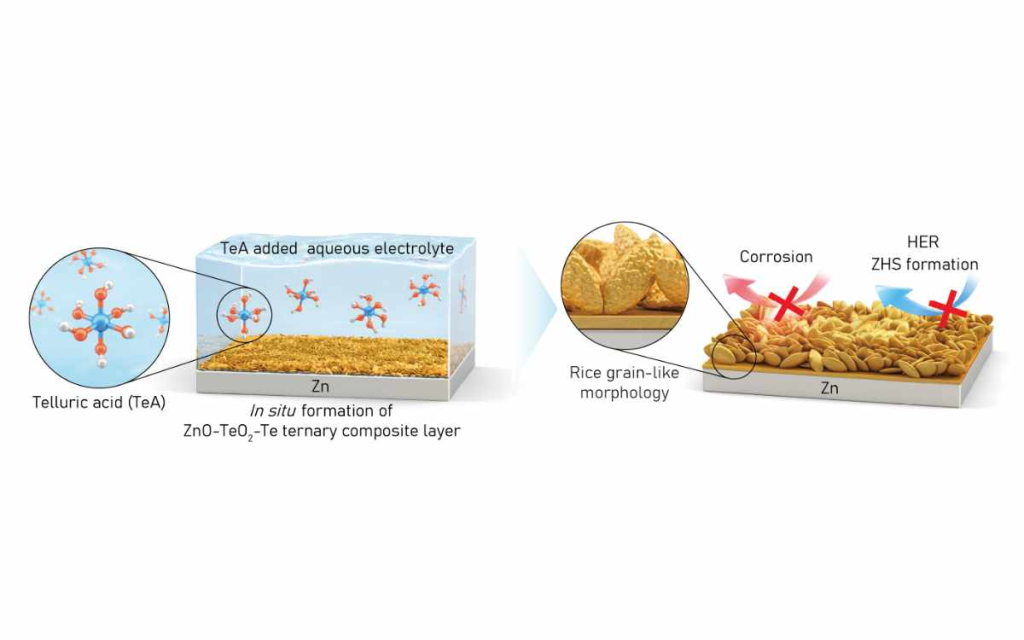
Zinc battery technology, particularly aqueous zinc batteries that use water-based electrolytes, offers benefits such as low fire risk, affordability and high capacity. However, they face challenges including electrode corrosion and byproduct accumulation. To address this, the team introduced telluric acid as an electrolyte additive, enabling the spontaneous formation of a ternary composite layer of zinc oxide (ZnO), tellurium dioxide (TeO₂), and tellurium (Te) on the electrode surface.
Analytical results confirmed the layer’s robust structure, with ZnO and TeO₂ on the surface and Te embedded within. The artificial layer suppressed dendrite growth and byproduct formation, allowing zinc to accumulate uniformly. Electrochemical analysis showed reduced corrosion-related resistance and improved ion mobility.
The zinc symmetric cell incorporating this protective layer operated stably for over 1,500 hours, outperforming conventional batteries under realistic conditions.
KU Professor Yu Seung-ho said, “We developed a method of forming a ternary composite artificial layer on an electrode surface using a telluric acid additive, thereby overcoming the weakness of zinc-based batteries. This is expected to be a key technology that will accelerate the commercialisation of next-generation eco-friendly batteries.”
The research was supported by Korea’s Ministry of Science and ICT and the National Research Foundation.